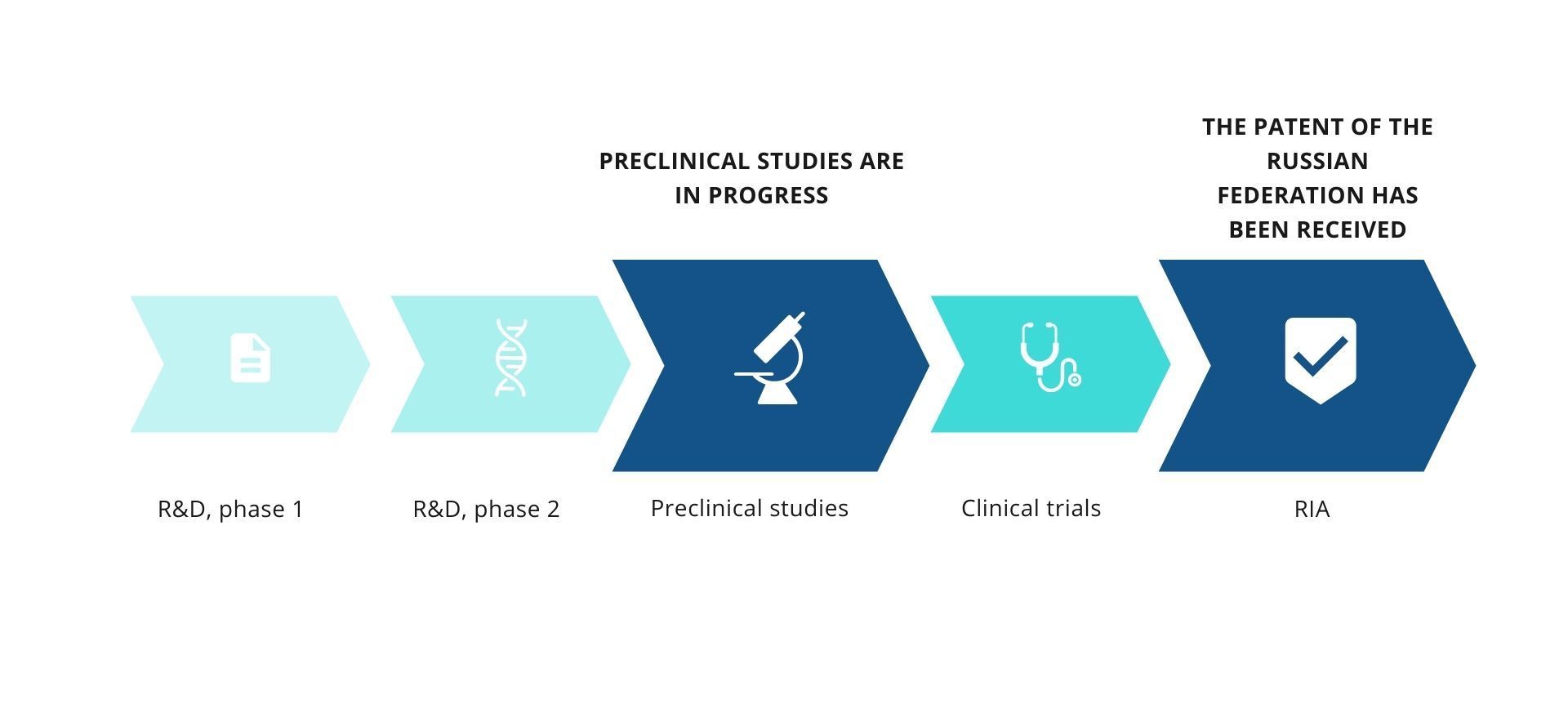
STAGE OF DEVELOPMENT
& RESULTS OF INTELLECTUAL ACTIVITY STATUS
EFFECT
Teriparatide stimulates the formation of bone tissue through a direct effect on osteoblasts.
APPLICATION
Used for the treatment of osteoporosis.
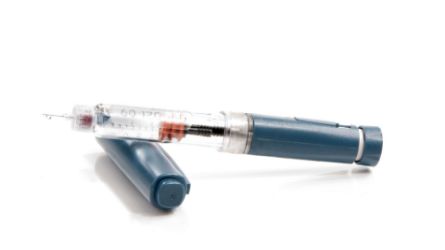
Description: Solution for subcutaneous administration, cartridge (syringe-pen).
LICENSES

FOR THE PRODUCTION OF MEDICINES
№ 11719-ЛС-П OF 25/06/2012
(VALIDITY PERIOD: UNLIMITED)

FOR STUDIES RELATED TO THE USE OF PATHOGENS OF INFECTIOUS DISEASES OF GROUPS III-IV PATHOGENICITY
(VALIDITY PERIOD: UNLIMITED)
DEVELOPERS
The Shemyakin & Ovchinnikov Institute of bioorganic chemistry (IBCh) of the Russian Academy of Sciences is one of the largest Russian scientific organizations. The Institute is a leader in fundamental and innovative researches on the fields of molecular, structural and cell biology, bioorganic chemistry, biophysics, bioengineering, cell technologies, “in vivo” molecular based bioimaging, genome editing, bioinformatics etc. This multidisciplinary structure allows large-scale research at the interface of sciences, where the most interesting scientific discoveries are born today.
The hallmark of the IBCh RAS is the concentration of efforts and resources on solving the most urgent and complicated problems on the field of life sciences. Talented young people and leading specialists, including Russian and foreign science leaders, Nobel Prize laureates and members of the international advisory council of the Institute are involved in.
PUBLICATIONS
M.M. Shemyakin and Yu.A. Ovchinnikov Institute of Bioorganic Chemistry RAS, 2021. С. 150.

