STAGE OF DEVELOPMENT
& RESULTS OF INTELLECTUAL ACTIVITY STATUS
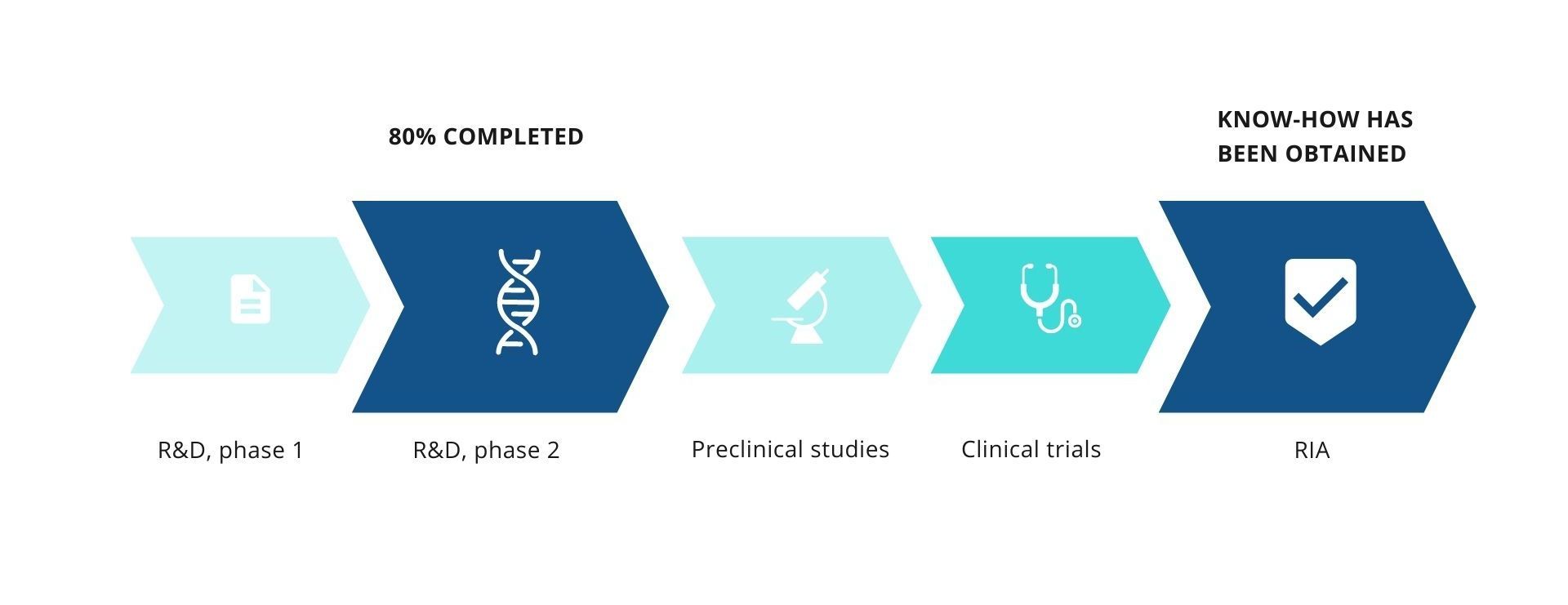
APPLICATION
Creation of new highly effective and safe drugs with antibacterial action for the treatment of hospital-acquired multidrug-resistant infections.
DEVELOPERS
The Shemyakin & Ovchinnikov Institute of bioorganic chemistry (IBCh) of the Russian Academy of Sciences is one of the largest Russian scientific organizations. The Institute is a leader in fundamental and innovative researches on the fields of molecular, structural and cell biology, bioorganic chemistry, biophysics, bioengineering, cell technologies, “in vivo” molecular based bioimaging, genome editing, bioinformatics etc. This multidisciplinary structure allows large-scale research at the interface of sciences, where the most interesting scientific discoveries are born today.
The hallmark of the IBCh RAS is the concentration of efforts and resources on solving the most urgent and complicated problems on the field of life sciences. Talented young people and leading specialists, including Russian and foreign science leaders, Nobel Prize laureates and members of the international advisory council of the Institute are involved in.
HEAD OF THE PROJECT
PUBLICATIONS
A kinase bioscavenger provides antibiotic resistance by extremely tight substrate binding.
Sci Adv. 2020 Jun 24;6(26):eaaz9861. doi: 10.1126/sciadv.aaz9861. PMID: 32637600; PMCID: PMC7314540.
Deep Functional Profiling Facilitates the Evaluation of the Antibacterial Potential of the Antibiotic Amicoumacin.
Terekhov SS, Nazarov AS, Mokrushina YA, Baranova MN, Potapova NA, Malakhova MV, Ilina EN, Smirnov IV, Gabibov AG.
Antibiotics. 2020 Apr 2;9(4):157. doi: 10.3390/antibiotics9040157. PMID: 32252356; PMCID: PMC7235827.
Ultrahigh-throughput functional profiling of microbiota communities.
Terekhov SS, Smirnov IV, Malakhova MV, Samoilov AE, Manolov AI, Nazarov AS, Danilov DV, Dubiley SA, Osterman IA, Rubtsova MP, Kostryukova ES, Ziganshin RH, Kornienko MA, Vanyushkina AA, Bukato ON, Ilina EN, Vlasov VV, Severinov KV, Gabibov AG, Altman S.
Proc Natl Acad Sci U S A. 2018 Sep 18;115(38):9551-9556. doi: 10.1073/pnas.1811250115. Epub 2018 Sep 4. PMID: 30181282; PMCID: PMC6156654.


